GnRH
tl;dr GnRH is a natural peptide used to “jumpstart” sex hormone production. You will not make testosterone without it’s pulse release in your brain. These effects are well-researched. Some new research is exploring what else it can do for brain aging and disease. GnRH can also be used to as PCT to reboot a broken HPTA.
Names: GnRH, GNRH, GnRF, Gonadorelin
Introduction
GnRH is Gonadotropin-Releasing Hormone. Depending on what you’re reading it’s also called FSH-RH, LHRH, or GnRF. While it is often categorized in the GHRP/GHRH series it really belongs in it’s class. The somewhat similar name doesn’t mean it does the same things.
Pulsatile action similar to hGH
GHRP/GHRH cause the release of hGH with a pulse release. GnRH works to release different hormones though. But GnRF does work in a pulse manner similar to these peptides.
Controls testosterone, sperm, and egg production
GnRH is a naturally occurring peptide. It is the prime mover to start many sexual functions. It binds to it’s own receptor (the gonadotropin-releasing hormone receptor) on the pituitary to start the release of luteinizing hormone (LH) and follicle-stimulating hormone (FSH). This in turn makes testosterone.
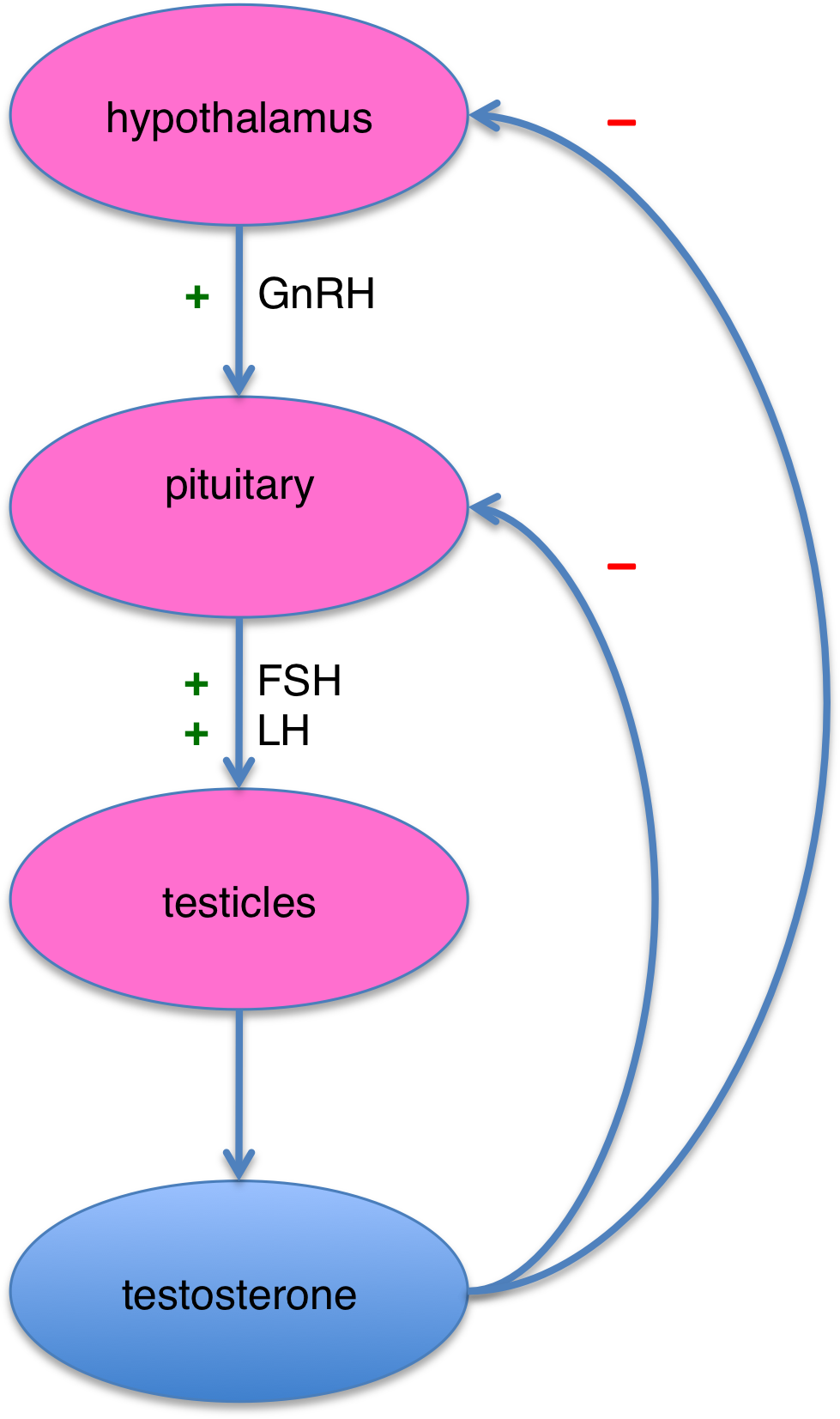
In this way it also acts a fertility hormone. Males and females with a GnRH problem are not going to be able to make sperm or eggs. And male testosterone generation is first controlled by GnRH secretion. Each gender’s release of GnRH is on different cycles as well.
Effective for PCT after steroid use
GnRH can be very useful to reboot a damaged HPTA axis or for PCT. Some anabolics will stop natural LH, FSH, and T production. GnRH can reboot this. You can read more about this in the PCT section.
“ GnRH production/release is one of the few confirmed examples of behavior influencing hormones, rather than the other way around.[citation needed] “” wikipedia, easy link
GnRH Medical Studies
Decades of medical research
GnRH is heavily research with studies dating back to the 1960’s detailing it’s effects on FSH, LH, and testosterone. The benefits of natural testosterone production, or replacement therapy TRT, for male performance are too obvious to mention here. And you can’t discuss women’s fertility without knowing GnRH’s role in production of FSH and LH.
So lets not re-explore 40 years worth of data about the relationship of GnRH to downstream hormones. It’s signaling effects can certainly be recreated by replacement. Instead are there effects that GnRH itself provides independent of other hormones?
What can GnRH do beside hormone signaling
Lets see how it does in keeping our goals in mind:
PTSD
Evidence:
Effect:
There are no studies testing GnRH as a PTSD cure/treatment. There are however several links between a two way relationship between stress and GnRH problems in humans and animals.
Stress situations and actual endocrine stress 1 both “negatively regulates GnRH neurons” 2 and directly “modulates GnRH secretion.” 3
In an animal model, social interactions and other stressful situations also had an inhibitory effect on GnRH production.4 The same applies to humans. When tested by blocking GnRH activity people reported having more mental distress.5
And as emotionally stressful situations also downregulate GnRH, there is a cyclical effect at play. The more stressed you get the worse your GnRH pathway functions. The worse your GnRH pathways function, the worse you feel.
Low theoretical effectiveness for PTSD
In a chronic PTSD situation with impaired GnRH production, GnRH injections or stimulation might offer an effective brake against endocrine issues. While not curing PTSD directly this might offer the person a chance at having a more normal endocrine system to bounce back from daily stress and begin tackling the root cause head on.
TBI
Evidence:
No effectiveness for TBIThere is no link between GnRH assisting recovery or life quality in TBI cases. TBI in children might require GnRH and hGH supplementation if there is local damage to the pituitary. 6
But in general, there is no studied effect of anything unique to GnRH for TBI recovery.
Alzheimer’s/Brain Aging
Evidence:
Effect:
Links to brain aging but not in a curative manner
GnRH activity and Alzheimer’s have some interplay. The exact mechanism is still a matter of debate.
Using GnRHr agonists
Optional Biochemistry Nerd Information: The way to test it is this: use a synthetic GnRH agonist (but NOT actual GnRH peptides.) This will cause a brief, but massive, LH/FSH spike. This is called a flare protocol. 789 Because it is so effective, the pituitary gland quickly downregulates GnRH receptors. After the flare’s effects wear off the receptors remain downregulated. The body is now affected much less by GnRH and it stays in a low LH/FSH/T state.
The biggest clue is the rise in LH seen at the start of Alzheimer’s and old age.1011 Since GnRH causes the release of LH, is there a broken feedback system at work? Could inhibiting GnRH - or normalizing it throughout old age - prevent this disease?
Some research seems to implicate GnRH is the worsening of Alzheimer’s.12 By blocking and limiting the effects of naturally occurring GnRH with an analog, parts (but not all) of the brain were somewhat less affected. But this was in a rat model and only offered partial protection.
Humans have a somewhat similar mechanism.13 At least in prostate cancer patients, this GnRH protocol caused less Alzheimer’s deaths. It also helps some post menopausal women from worsening with the disease as well.14
GnRH itself seems secondary to any brain aging
GnRH itself seems to be implicated in the progression of the disease. Halting or slowing down the natural effects of GnRH seems to the be way to go. While GnRH itself does not cause anything bad, it’s actions in the pituitary to cause LH/FSH release might be to blame. Based on that, low level GnRH use would be perhaps the worst possible thing for a dementia/Alzheimer patient to do.
Neuroprotective Effects
Evidence:
Effect:
There is weak evidence that GnRH has neuroprotective effects. An Italian study shows that there is a “possible role of GnRH in neuronal functions”15 outside of just sex hormone release. The authors think it can “suggest a possible mechanism of GnRH-induced neuroprotection” but the “exact role in different brain structures has not been completely defined.”Theoretical use of GnRH inside the brain
The evidence is that there are some GnRH receptors deep in the brain and on some motor neurons. There would be no reason for these receptors to have evolved there if GnRH was not providing some effect there.
Nerve Regrowth
Evidence:
Effect:
Several studies point to GnRH as having positive effects for spinal cord injury recovery.
In the most promising study a GnRH agonist was shown to improve functions after spinal cord compression injury in rats after 5 weeks. They recovered much better compared to the control group without GnRH activation. 16
The effects are measurable and real. The rats with damaged spinal cords had a better gait and nerve regrowth after treatment. 17
A similar study shows how the GnRH receptor in spinal cord neurons play a role in neuronal plasticity.18 This would also mean that GnRH could help heal spinal cord injuries by stimulating growth.
GnRH could heal nerve damage…
A big question from these studies is if natural GnRH ever makes it to the injury in the first place. Perhaps the forced over-activation on GnRHR triggers a central nervous system repair? If this is true, using GnRH for non-severing spinal injuries could help recover lost functions in human.
This works with a pet theory I have: that the spinal cord is capable of magnified healing. For whatever reason though, perhaps distance from the brain, nerve regrowth factors simply don’t reach it. This causes reduced healing. This (along with many other reasons) also makes it impossible for autophagy or Schwann cell recruitment to take place.
…but synthetic analogs would be better suited
Many studies use synthetic GnRH analogs (ex: leuprolide acetate) that are super potent and long lasting. Compare this to the natural fragile peptide with a 5 minute half-life that must somehow travel across the spine. Perhaps future trials of GnRH analogs could finally allow for completely severed spinal cord recovery.
Outside of the central nervous system though, GnRH does not appear to be used for any peripheral nerve growth, repair, or regeneration. A very similar peptide GnRH II is used in the peripheral nervous system though.19
Muscle Growth
Evidence:
The only direct link GnRH has to muscle growth or repair is in signaling T production. Smooth muscles have a GnRH receptor.20 But skeletal muscles themselves do not respond to GnRH use.GnRH as a TRT Replacement
Can GnRH be used a replacement for TRT for secondary hypogonadism? Instead of using just T which can halt LH/FSH production and cause infertility, perhaps GnRH could be used instead to naturally elevate levels of all 3.
Needs very long term studies
It will be 40 years before guys in their 30s on TRT now start starting entering older age. Would pulsatile GnRH have been better because there is a certain something in it that just testosterone + hCG can’t provide?
Unfortunately the use of GnRH past 1-2 years is not in any medical studies. And having a pump deliver GnRH every 90 minutes for life is more trouble than an E3D/E7D shot.
GnRH is Stress Sensitive
GnRH levels are related to external and internal stress
GnRH production is sensitive to stress. Both in the bio-chemical sense and the psychological sense.
Environment stress situations are shown to reduce GnRH release. Much of the research is done on animal breeding. For example in birds and rodents specifically it is believed GnRH inhibitory hormones “may serve as a transducer of environmental information and social interactions.”21 Similar effects are seen in fish breeding with social stress causing endocrine stress via cortisol.22
Many different stressors can reduce GnRH
Humans are also affected because we can release a hormone designed just to inhibit GnRH as well. This hormone is called gonadotropin-inhibitory hormone (GnIH) and functions as a brake on, ultimately, Testosterone production. It can be so strong that it can cause infertility.23
As in other animals this could be due to a sensitive social feedback loop, or the stress/inflammatory pathways in the body. Specifically IKK-β and NF-κB seem to implicated in inhibition of GnRH.
It seems the easiest way to naturally boost GnRH levels and keep it effective is to be less stressed. This avoids the negative feedback loops.
GnRH for PCT
Evidence:
Effect:
Many people discuss the role of GnRH for PCT. The big problem becomes figuring out the exact peptide/protocol used.Useful, but timing is important
Natural GnRH and GnRH drug analogs can get the same effect but behave very differently. Natural GnRH is released in pulses approximately every 90 minutes and has a very short half-life - just a few minutes.24 At natural doses there is no downregulation.
Natural GnRH supplementation is very effective:
Pulsatile GnRH treatment fixes HPTA axis problems and restarts T production. 25
Pulsatile GnRH at 90 minute intervals restored fertility, ovulation, and allowed for pregnancy in women who did not respond to Clomid.26
The same pulses of 5-20 micrograms of GnRH every 89 minutes made men fertile enough to father children again.27
GnRH peptides vs synthetic analogs like Triptorelin for PCT
Drug analogs can have much longer half-lifes, often lasting hours.28 They also can bind for much longer and overwhelm GnRH receptors because their binding capacity can be more than 100x that of GnRH itself.29 This could cause all kinds of sensitivity issues that can take weeks to recover from if not monitored. Not a fun thing to have on top of an already possibly out of sync HPTA axis.
(You can read more about synthetic GnRH analog use in the optional Biochemistry Nerd section of GnRH for Alzheimer’s disease treatment above)
There is an exciting report of how Italian doctors successfully restarted the HPTA axis of a patient.30 His LF/FSH/T levels rebounded after 13 years of steroid use. All it took was one injection of the GnRH agonist Triptorelin at 100mcg to get his pituitary working again. T levels went back to normal then.
Single shot for PCT…
This study has been the rationale behind claiming GnRH/Triptorelin is a “one and done” fix for bodybuilding PCT.
The studies show that for someone who is in HPTA shutdown GnRH or triptorelin acetate could result in recovery. It was just an N=1 report though. For bodybuilding it seems people report it working about 50% of the time. Unfortunately this research is suffering for lack of access to pure Triptorelin.
…but it often isn’t that easy
In many cases the use of GnRH is confused with an analog. Or the triptorelin’s source is questionable, or the PCT is run on top of Clomid/hCG. Or, even worse, people think that Triptorelin is GnRH itself. Since the two have dosing differences of 10-20x this is dangerous.
Triptorelin would best be prescribed by a doctor to ensure getting an accurate product. If after 1 or 2 100mcg doses the blood work doesn’t come back normal though, there is something else going on preventing T production.
Using just GnRH peptide itself like your body does
Pure GnRH peptides should also restart sex hormone production. The downside is mimicking the 90 minute pulse effects and getting the dose right: natural GnRH works at the 5-10mcg level for restoring testical functions.31
Instead of multiple injections throughout the day of GnRH peptides, modifying a diabetes pump would be more effective. It will automatically release the correct amounts on schedule.
Overdoing it can cause severe problems
But the effectiveness of GnRH therapy can come at a cost. Is it possible to overdo it though? Yes.
The fact is any GnRH analog like Triptorelin gives a nice, healthy jumpstart to sex hormone production.
Continuous use though will cause suppression of the same hormones. Overloading your GnRH system is, for all intents, chemical castration - German sex offenders are being chemically castrated using GnRH agonists.32. Triptorelin is used to PREVENT puberty from starting.33 Long term GnRH analogs also had a 94% success rate in chemical castration.34
This is the complete opposite of PCT.
Patents
Patents for GnRH are mostly focused on the downregulation/de-sensitization aspects. Blocking GnRH functions leads to a massive drop in sex-hormone levels. Certain cancers like breast or prostate cancer are sensitive to these. By dropping sex-hormone levels these cancers can be treated easier.
Patent US20150231053A1: Treatment of skin, including aging skin, to improve appearance uses, among other hormones, GnRH for better skin tone.
Dosing
- 5-10mcg every ~90 minutes mimics natural human production. The constant pulse release of GnRH is needed for it’s effects.
- A single 100mcg injection of GnRH analog Triptorelin for PCT
- 10 mcg/kg has been tested in rats
Pricing
GnRH as a reference chemical is sold either as analogs (ex: Triptorelin) or as the pharmaceutical name Gonadorelin. Peptide sites often sell Triptorelin and GnRH interchangeable. Their mass spectrometry results should show that Triptorelin is C64H82N18O13, GnRH is C55H75N17O13 if they aren’t showing a difference.
Peptide site pricing:
- GnRH: $25-$42 for 2000mcg/2mg
- Triptorelin: $30-$36 for 100mcg
Chemical vendor pricing:
- Gonadorelin: $54-138 for 5mg, $310 for 250mg
- Triptorelin: $210 for 100mg
References
- Clarke, Iain J., Danielle Bartolini, Gregory Conductier, and Belinda A. Henry. 2016. “Stress Increases Gonadotropin Inhibitory Hormone Cell Activity and Input to GnRH Cells in Ewes.” Endocrinology 157 (11) (November): 4339–4350. doi:10.1210/en.2016-1513. http://dx.doi.org/10.1210/en.2016-1513. ↵
- Kim, Helen. 2007. “Regulation of Gonadotropin-Releasing Hormone Gene Expression.” Seminars in Reproductive Medicine 25 (5) (September): 313–325. doi:10.1055/s-2007-984737. http://dx.doi.org/10.1055/s-2007-984737. ↵
- X. F. Li, A. M. I. Knox, and K. T. O’Byrne, “Corticotrophin-releasing factor and stress-induced inhibition of the gonadotrophin-releasing hormone pulse generator in the female,” Brain Research, vol. 1364, pp. 153–163, Dec. 2010 [Online]. Available: http://dx.doi.org/10.1016/j.brainres.2010.08.036 ↵
- R. Ullah, Y. Shen, Y. Zhou, K. Huang, J. Fu, F. Wahab, and M. Shahab, “Expression and actions of GnIH and its orthologs in vertebrates: Current status and advanced knowledge,” Neuropeptides, vol. 59, pp. 9–20, Oct. 2016 [Online]. Available: http://dx.doi.org/10.1016/j.npep.2016.05.004 ↵
- D. S. Stenbaek, M. Toftager, L. V. Hjordt, P. S. Jensen, K. K. Holst, T. Bryndorf, T. Holland, J. Bogstad, A. Pinborg, P. Hornnes, and V. G. Frokjaer, “Mental distress and personality in women undergoing GnRH agonist versus GnRH antagonist protocols for assisted reproductive technology,” Human Reproduction, vol. 30, no. 1, pp. 103–110, Nov. 2014 [Online]. Available: http://dx.doi.org/10.1093/humrep/deu294 ↵
- Casano-Sancho, Paula, Larisa Suárez, Lourdes Ibáñez, Gemma García-Fructuoso, Julita Medina, and Anna Febrer. 2013. “Pituitary Dysfunction after Traumatic Brain Injury in Children: Is There a Need for Ongoing Endocrine Assessment?” Clinical Endocrinology 79 (6) (June 27): 853–858. doi:10.1111/cen.12237. http://dx.doi.org/10.1111/cen.12237. ↵
- Zhang, Hong, Ya-Qiong Liu, Guang-Xiu Lu, and Fei Gong. 2016. “Live Birth in a 46-Year-Old Woman Using Microdose GnRH Agonist Flare-up Protocol Combined with GnRH Antagonist: a Case Report.” Clinical Case Reports 4 (12) (October 12): 1107–1111. Portico. doi:10.1002/ccr3.693. http://dx.doi.org/10.1002/ccr3.693. ↵
- Orvieto, Raoul. 2015. “A Simplified Universal Approach to COH Protocol for IVF: Ultrashort Flare GnRH-agonist/GnRH-Antagonist Protocol with Tailored Mode and Timing of Final Follicular Maturation.” Journal of Ovarian Research 8 (1) (November 4). doi:10.1186/s13048-015-0198-3. http://dx.doi.org/10.1186/s13048-015-0198-3. ↵
- Growth Hormone Supplementation in the Luteal Phase Before Microdose GnRH Agonist Flare Protocol for In Vitro Fertilization. Dunne C, Seethram K, Roberts J., J Obstet Gynaecol Can. 2015 Sep; 37(9):810-5. [PMID:26605451] ↵
- Burnham, Veronica L., and Janice E. Thornton. 2015. “Luteinizing Hormone as a Key Player in the Cognitive Decline of Alzheimer’s Disease.” Hormones and Behavior 76 (November): 48–56. doi:10.1016/j.yhbeh.2015.05.010. http://dx.doi.org/10.1016/j.yhbeh.2015.05.010. ↵
- Webber, Kate M., Gemma Casadesus, Craig S. Atwood, Richard L. Bowen, George Perry, and Mark A. Smith. 2007. “Gonadotropins: A Cohesive Gender-Based Etiology of Alzheimer Disease.” Molecular and Cellular Endocrinology 260-262 (January): 271–275. doi:10.1016/j.mce.2006.01.018. http://dx.doi.org/10.1016/j.mce.2006.01.018. ↵
- Nuruddin, Syed, Gry Helen Enger Syverstad, Sveinung Lillehaug, Trygve B. Leergaard, Lars N. G. Nilsson, Erik Ropstad, Anette Krogenæs, Ira Ronit Hebold Haraldsen, and Reidun Torp. 2014. “Elevated mRNA-Levels of Gonadotropin-Releasing Hormone and Its Receptor in Plaque-Bearing Alzheimer’s Disease Transgenic Mice.” Edited by Andrew Wolfe. PLoS ONE 9 (8) (August 4): e103607. doi:10.1371/journal.pone.0103607. http://dx.doi.org/10.1371/journal.pone.0103607. ↵
- DʼAmico, Anthony V., Michelle H. Braccioforte, Brian J. Moran, and Ming-Hui Chen. 2010. “Luteinizing-Hormone Releasing Hormone Therapy and the Risk of Death From Alzheimer Disease.” Alzheimer Disease & Associated Disorders 24 (1) (January): 85–89. doi:10.1097/wad.0b013e31819cb8f4. http://dx.doi.org/10.1097/WAD.0b013e31819cb8f4. ↵
- Casadesus, Gemma, Matthew R Garrett, Kate M Webber, Anthony W Hartzler, Craig S Atwood, George Perry, Richard L Bowen, and Mark A Smith. 2006. “The Estrogen Myth.” Drugs in R & D 7 (3): 187–193. doi:10.2165/00126839-200607030-00004. http://dx.doi.org/10.2165/00126839-200607030-00004. ↵
- Maggi R (2016) Physiology of Gonadotropin-Releasing Hormone (Gnrh): Beyond the Control of Reproductive Functions. MOJ Anat & Physiol 2(5): 00063. DOI: 10.15406/mojap.2016.02.00063 ↵
- Quintanar, Jluis, Carmen Díaz-Galindo, Beatriz Gómez-González, Eva Salinas, Denisse Calderón-Vallejo, Irma Hernández-Jasso, and Eduardo Bautista. 2015. “Leuprolide Acetate Induces Structural and Functional Recovery of Injured Spinal Cord in Rats.” Neural Regeneration Research 10 (11): 1819. doi:10.4103/1673-5374.170311. http://dx.doi.org/10.4103/1673-5374.170311. ↵
- Calderón-Vallejo, Denisse, Andrés Quintanar-Stephano, Irma Hernández-Jasso, Violeta Jiménez-Hernández, Jannet Ruiz-Ornelas, Ismael Jiménez, and J. Luis Quintanar. 2015. “Functional and Structural Recovery of the Injured Spinal Cord in Rats Treated with Gonadotropin-Releasing Hormone.” Neurochemical Research 40 (3) (January 25): 455–462. doi:10.1007/s11064-014-1486-9. http://dx.doi.org/10.1007/s11064-014-1486-9. ↵
- Quintanar, J. Luis, Denisse Calderón-Vallejo, and Irma Hernández-Jasso. 2016. “Effects of GnRH on Neurite Outgrowth, Neurofilament and Spinophilin Proteins Expression in Cultured Spinal Cord Neurons of Rat Embryos.” Neurochemical Research 41 (10) (June 23): 2693–2698. doi:10.1007/s11064-016-1983-0. http://dx.doi.org/10.1007/s11064-016-1983-0. ↵
- Millar, Robert P. 2003. “GnRH II and Type II GnRH Receptors.” Trends in Endocrinology & Metabolism 14 (1) (January): 35–43. doi:10.1016/s1043-2760(02)00016-4. http://dx.doi.org/10.1016/S1043-2760(02)00016-4. ↵
- Chegini, N, H Rong, Q Dou, S Kipersztok, and R S Williams. 1996. “Gonadotropin-Releasing Hormone (GnRH) and GnRH Receptor Gene Expression in Human Myometrium and Leiomyomata and the Direct Action of GnRH Analogs on Myometrial Smooth Muscle Cells and Interaction with Ovarian Steroids in Vitro.” The Journal of Clinical Endocrinology & Metabolism 81 (9) (September): 3215–3221. doi:10.1210/jcem.81.9.8784072. http://dx.doi.org/10.1210/jcem.81.9.8784072. ↵
- Ubuka, T., N. L. McGuire, R. M. Calisi, N. Perfito, and G. E. Bentley. 2008. “The Control of Reproductive Physiology and Behavior by Gonadotropin-Inhibitory Hormone.” Integrative and Comparative Biology 48 (5) (April 19): 560–569. doi:10.1093/icb/icn019. http://dx.doi.org/10.1093/icb/icn019. ↵
- Korzan, W. J., B. P. Grone, and R. D. Fernald. 2014. “Social Regulation of Cortisol Receptor Gene Expression.” Journal of Experimental Biology 217 (18) (July 10): 3221–3228. doi:10.1242/jeb.104430. http://dx.doi.org/10.1242/jeb.104430. ↵
- Nargund, Vinod H. 2015. “Effects of Psychological Stress on Male Fertility.” Nature Reviews Urology 12 (7) (June 9): 373–382. doi:10.1038/nrurol.2015.112. http://dx.doi.org/10.1038/nrurol.2015.112. ↵
- Hayden, C. 2008. “GnRH Analogues: Applications in Assisted Reproductive Techniques.” European Journal of Endocrinology 159 (suppl_1) (October 10): S17–S25. doi:10.1530/eje-08-0354. http://dx.doi.org/10.1530/EJE-08-0354. ↵
- Dysfunction of hypothalamic-pituitary-testicular axis in patients with adrenal hypoplasia congenita due to DAX-1 gene mutation. Article in Chinese Zhonghua Yi Xue Za Zhi. 2016 Apr 19; 96(15):1183-7. [PMID:27117364] https://www.ncbi.nlm.nih.gov/pubmed/27117364 ↵
- Hurley, David M., Rhonda Brian, Ken Outch, Jan Stockdale, Annette Fry, Charles Hackman, Iain Clarke, and Henry G. Burger. 1984. “Induction of Ovulation and Fertility in Amenorrheic Women by Pulsatile Low-Dose Gonadotropin-Releasing Hormone.” New England Journal of Medicine 310 (17) (April 26): 1069–1074. doi:10.1056/nejm198404263101702. http://dx.doi.org/10.1056/NEJM198404263101702. ↵
- Blumenfeld, Z., A. Makler, L. Frisch, and J. M. Brandes. 1988. “Induction of Spermatogenesis and Fertility in Hypogonadotropic Azoospermic Men by Intravenous Pulsatile Gonadotropin-Releasing Hormone (GnRH).” Gynecological Endocrinology 2 (2) (January): 151–164. doi:10.3109/09513598809023623. http://dx.doi.org/10.3109/09513598809023623. ↵
- Müller, F. O., J. Terblanchè, R. Schall, R. Van Zyl Smit, T. Tucker, K. Marais, G. Groenewoud, H. C. Porchet, M. Weiner, and D. Hawarden. 2003. “Pharmacokinetics of Triptorelin after Intravenous Bolus Administration in Healthy Males and in Males with Renal or Hepatic Insufficiency.” British Journal of Clinical Pharmacology 44 (4) (October 2): 335–341. doi:10.1046/j.1365-2125.1997.t01-1-00592.x. http://dx.doi.org/10.1046/j.1365-2125.1997.t01-1-00592.x. ↵
- Hayden, C. 2008. “GnRH Analogues: Applications in Assisted Reproductive Techniques.” European Journal of Endocrinology 159 (suppl_1) (October 10): S17–S25. doi:10.1530/eje-08-0354. http://dx.doi.org/10.1530/EJE-08-0354. ↵
- Pirola, Ilenia, Carlo Cappelli, Andrea Delbarba, Tiziano Scalvini, Barbara Agosti, Deodato Assanelli, Antonio Bonetti, and Maurizio Castellano. 2010. “Anabolic Steroids Purchased on the Internet as a Cause of Prolonged Hypogonadotropic Hypogonadism.” Fertility and Sterility 94 (6) (November): 2331.e1–2331.e3. doi:10.1016/j.fertnstert.2010.03.042. http://dx.doi.org/10.1016/j.fertnstert.2010.03.042. ↵
- Gong, Chunxiu, Ying Liu, Miao Qin, Di Wu, and Xiaoling Wang. 2015. “Pulsatile GnRH Is Superior to hCG in Therapeutic Efficacy in Adolescent Boys With Hypogonadotropic Hypogonadodism.” The Journal of Clinical Endocrinology & Metabolism 100 (7) (July): 2793–2799. doi:10.1210/jc.2015-1343. http://dx.doi.org/10.1210/jc.2015-1343. ↵
- Turner, Daniel, Raphaela Basdekis‐Jozsa, and Peer Briken. 2013. “Prescription of Testosterone‐Lowering Medications for Sex Offender Treatment in German Forensic‐Psychiatric Institutions.” The Journal of Sexual Medicine 10 (2) (February): 570–578. doi:10.1111/j.1743-6109.2012.02958.x. http://dx.doi.org/10.1111/j.1743-6109.2012.02958.x. ↵
- Schagen, Sebastian E.E., Peggy T. Cohen-Kettenis, Henriette A. Delemarre-van de Waal, and Sabine E. Hannema. 2016. “Efficacy and Safety of Gonadotropin-Releasing Hormone Agonist Treatment to Suppress Puberty in Gender Dysphoric Adolescents.” The Journal of Sexual Medicine 13 (7) (July): 1125–1132. doi:10.1016/j.jsxm.2016.05.004. http://dx.doi.org/10.1016/j.jsxm.2016.05.004. ↵
- Dragnic, Sanja, Aaron Spitz, Marc Gittelman, Lawrence Karsh, Ahmed Soliman, Aditya Lele, Damian Gruca, and Michael Norton. 2016. “Intramuscular Depot Formulations of Leuprolide Acetate Suppress Testosterone Levels Below a 20 ng/dL Threshold: a Retrospective Analysis of Two Phase III Studies.” Research and Reports in Urology Volume 8 (August): 159–164. doi:10.2147/rru.s111475. http://dx.doi.org/10.2147/RRU.S111475. ↵
Last updated: